Abstract
Introduction: More than half of patients with myelodysplastic syndrome (MDS) have spliceosome mutations (SM) including ZRSR2, U2AF1, SF3B1 and SRSF2. Splicing is fundamental for proper mRNA processing. Mechanistic descriptions of the after-effects of SM remain elusive as improperly formed mRNA quickly decays or changes are subtle and may lack observable effects. These mutations have been implicated in multiple biological alterations including myeloid proliferation through aberrant NF-kB signaling. SRSF2P95* has been consistently shown to provide the highest fitness advantage in the bone marrow environment. This study reports the characteristics, treatment outcomes, leukemia free and overall survival of MDS with spliceosome mutations.
Patients and Methods: A retrospective review of patients diagnosed with MDS older than 18 for whom genomic data was available were included at a single institution. Patient characteristics and bone marrow data, including cytogenetic and next generation sequencing (NGS) information were assessed. Genomic DNA was extracted from whole bone marrow aspirate samples and subject to 81-gene target PCR-based sequencing using a NGS platform. The IWG 2006 response assessment criteria were used. Overall and leukemia-free survival were calculated, the latter as a composite of acute myeloid leukemia diagnosis or death from any cause, using the log-rank test for group comparison.
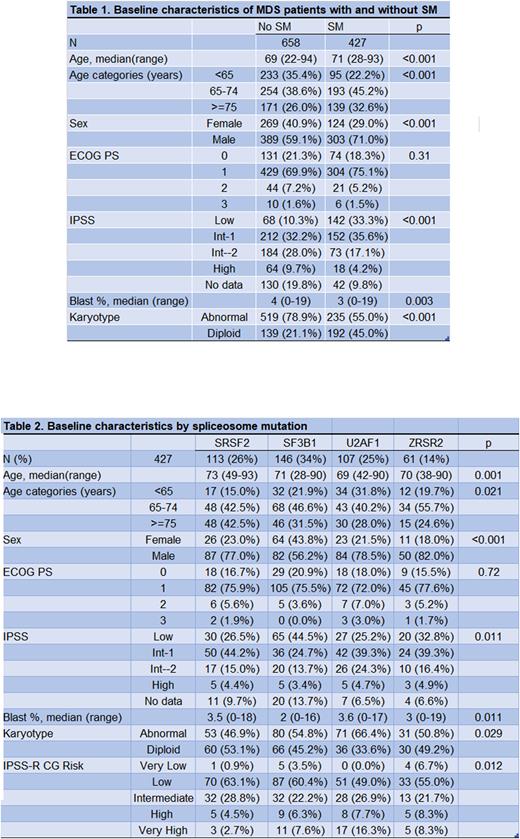
Results: First, we compared patients with SM vs no SM. We identified 427 patients with SM and 658 patients with no SM from 2017 to 2022 (Table 1). Among patients with SM, 71% were male, median age was 71. SM patients had higher incidence of IPSS Low (33.3% vs 10.3%) and IPSS Int- 1 (35.6% vs 32.2%), and a higher incidence of diploid karyotype (45% vs 21%), compared to patients with no SM. There was no difference in treatment regimens, and 66.9% of patients with SM received hypomethylating agents (HMA) alone or in combination vs 60.3% in the group without SM. MDS with SM had a lower CR/CRi rate (27% vs 36%); however, they had higher hematologic improvement (HI) (23% vs 14%) and lower chance of disease progression or death at the time of response assessment (39% vs 31% (p = 0.01)). Leukemia-free survival was longer in patients with SM vs no SM (median 4.1 years vs 1.9 years (p<0.0001), this was independent of SF3B1 mutations. Median OS was 4.5 years vs 1.9 years in patients with SM and no SM, respectively (p <0.0001).
We then compared outcomes between patients with specific SM mutations. These included SF3B1 (34%), SRSF2 (26%), U2AF1 (25%), and ZRSR2 (14%). Thirty-six percent were female. Median age was 71 (range 28-93). Median percentage of blasts was 3 (range 0-19). Most frequent diagnoses were MDS-EB (35%), MDS-RS (25%) and MDS-MLD (23%). IPSS was Low, Intermediate 1, Intermediate 2 and High in 33.3%, 35.6%, 17.1% and 4.2%, respectively. Fifty-seven percent were in the favorable cytogenetic risk group, 44% with diploid karyotype. Patients with a SM mutation did not share any other SM mutation (Table 2). There were no differences in therapy selected or response rate among SM mutations. Median LFS was not reached (NR), 5.7 years, 3.4 years, and 2.7 years for patients with SF3B1, ZRSR2, U2AF1, and SRSF2 mutations, respectively. Median OS was NR, 5.7 years, 3.6 years, and 2.7 years (p = 0.005) for the same groups, respectively.
Conclusions: In our study, patients with SM had a lower rate of CR/CRi with HMA-based therapy, however, leukemia free and overall survival were markedly different for each mutated gene. SM mutations were significantly more prevalent in males. Compared to MDS with diploid cytogenetics, ZRSR2 and SF3B1 mutations have a similar median LFS and OS, but SRSF2 and U2AF1 mutations carry a significantly worse median LFS and OS. While it is convenient to group these mutations together, prognostication should be individualized to specific mutations and co-mutations.
Disclosures
Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria. Kanagal-Shamanna:Amgen: Consultancy; Novartis: Consultancy; Aptitude Health: Speakers Bureau; Physicians Education Resource: Speakers Bureau. Kantarjian:ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Garcia-Manero:AbbVie: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Curis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Gilead Sciences: Research Funding; Novartis: Honoraria, Research Funding; Aprea: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.